Adam R. Johnson Research
If you are interested in working with Prof. Johnson, please email him at adam_johnson@hmc.edu to set up an appointment to discuss the research opportunities in more detail.
Organometallic Chemistry and Asymmetric Catalysis
My research involves the design and synthesis of amino alcohol ligands with tunable steric and electronic properties in order to develop better organometallic catalysts for interesting organic transformations. We use the standard techniques of organic synthesis as well a glove box or Schlenk line for working with air sensitive transition metal complexes. We use our complexes for catalytic asymmetric hydroamination. We study these reactions by variable temperature one- and two-dimensional NMR spectroscopy, kinetics experiments, and theoretical modeling. We are currently engaged in three main avenues of research: synthesis of new ligands, synthesis of new substrates, and mechanistic studies.
Synthesis of chiral ligands from amino acids: Chiral amino alcohols can be synthesized starting from commercially available amino acids. The syntheses are short (3-5 steps, typically), high-yielding, and use a variety of important organic transformations such as reductive amination, Grignard alkylation, and amide bond formation. Some of ligands my group is currently targeting are shown here. This project will involve synthesis of new ligands, coordination to Ti and/or Ta precursors and catalytic hydroamination.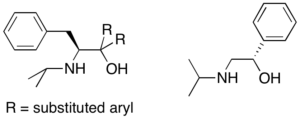
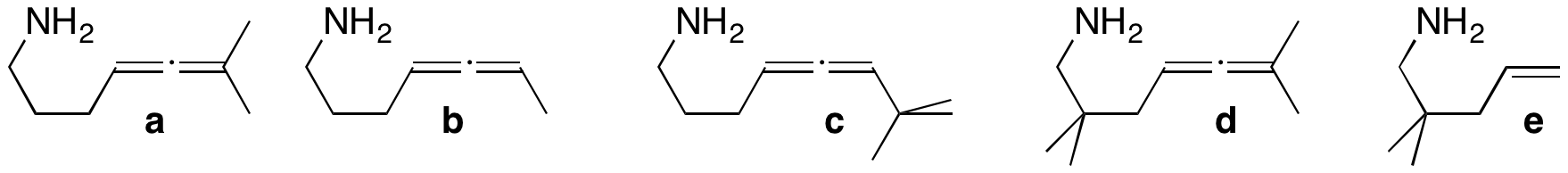
Synthesis of new hydroamination substrates. Almost all of our hydroamination work has used substrate a. Although some of our earlier work examined substrate b, we need to go back and look in more detail at the catalysis using that substrate; we may be able to observe kinetic resolution of the chiral (racemic) b. Substrate c is larger and should show selectivity. Substrate d has gem-dialkyl substituents which should allow our catalytic reaction to occur at a lower temperature. We will also investigate the hydroamination of aminoalkenes (such as e). The substrates are synthesized in 5-8 steps.
Catalytic hydroamination. Titanium (and tantalum) complexes catalyze the intramolecular hydroamination (addition of an N-H bond across a C-C multiple bond) of 1,3-aminoallenes as shown here. Our complexes form product 1 in high yield (100% when R’ = Me), and the enantiomeric excess is low (ca. 16%) for Ti, but much higher (ca. 75%) for Ta. We test all new complexes and substrates in the hydroamination reaction.
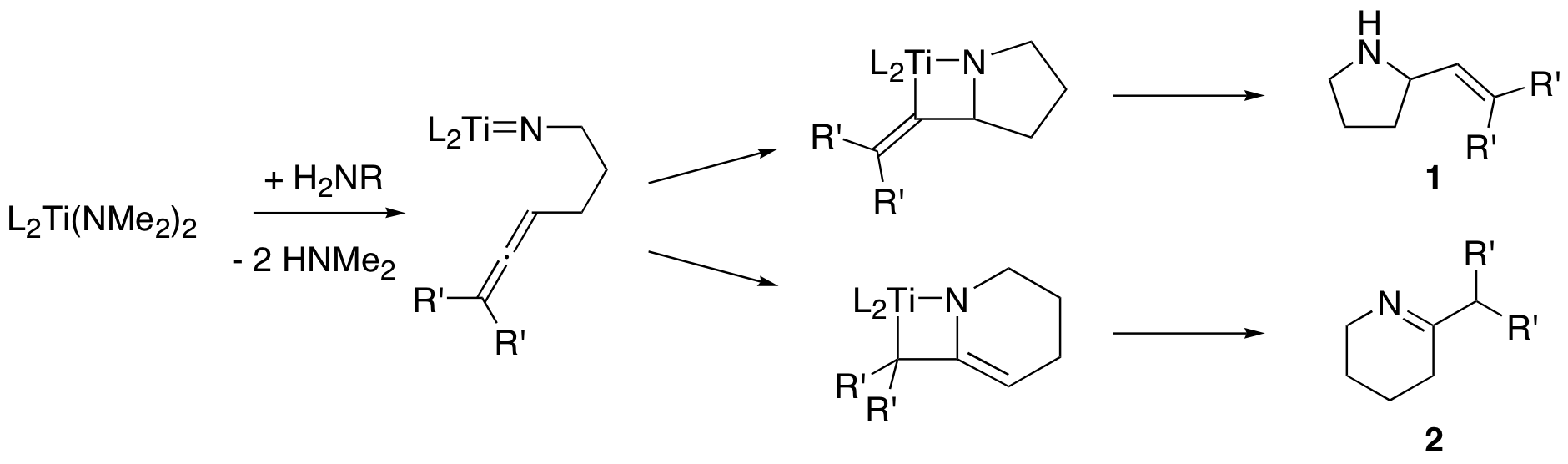
Mechanism. In our recent work, several important questions have come up. We need to do kinetics experiments to answer some of them. Other questions will involve physical inorganic chemistry (various forms of spectroscopy and other measurements). Some of the questions we want to answer include: are the Ti and Ta derived catalysts monomeric or dimeric in solution? What is the rate of the reaction? Does free amine coordinate to the metal during catalysis? Does the mechanism involve a [2+2] cycloaddition (shown above) or a 1,2-insertion reaction? Is the enantioselectivity of the reaction constant with time, or does it change?
For more information, read these papers (I’ll email them if you ask):
Organometallics,2011,30, 4616-4623
J. Organometal. Chem.,2011,696, 81-86
Tetrahedron: Asymmetry,2009,20, 1279-1285
Organometallics,2004,23, 4614-4620